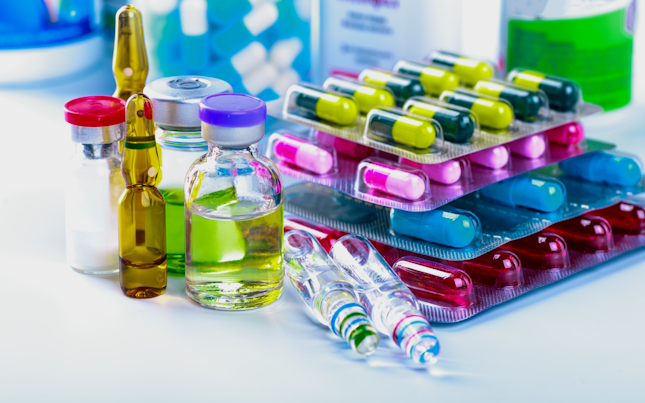
Comprehensive range of world-leading cGMP pharmaceutical product testing services delivered by high performance teams using state-of-the-art technologies across 19 facilities in 10 countries.
Integrity and scientific rigor are vital to ensure you have timely and accurate results on which to make informed, data-led decisions.
With a global network of industry experts, we offer a full range of cGMP pharmaceutical testing solutions that conform to international regulatory standards. Our specialists offer flexibility and provide additional technical support, helping you to meet your evolving business needs. All services are delivered with integrity and accuracy through clear lines of communication.
Our range of solutions can be delivered full-time or when you require extra flexibility during periods of excessive workload. No matter what your industry, product or sample, our focus is always on delivering accurate and timely results that help you make informed broader product quality decisions.
Looking for something specific?
Search within Pharmaceutical Testing
Connectivity & Products (Functional Safety)
Connectivity & Products (Product Safety/EMC)
Connectivity & Products (RSTS)
Connectivity & Products (Wireless)
Business Assurance (Management System Certification)
Business Assurance (Sustainability Services)
Business Assurance (Medical Device Certification)
Business Assurance (Forestry Certification)
Business Assurance (Food Certification)
Business Assurance (Marketing & Training)
Health & Nutrition (Food, Cosmetics & Hygiene)
Oil, Gas & Chemicals
Natural Resources
Industries
134, Godo-cho, Hodogaya-ku,
Yokohama Business Park North Square I 5F, 240-0005,
Yokohama, Japan